How researchers test new treatments, like hydroxychloroquine

In the beginning of a new disease, pretty much everything is unknown. Remember when everyone was calling this strange bug the "novel" coronavirus? Novel means new, meaning we had no idea what we were dealing with. We didn't know how COVID-19 was spread, how long it lasted or how it affected the body. This is where science comes in handy.
Trained, experienced researchers use the process of science to add knowledge to the collective understanding of how something works. Every treatment goes through the same process to see if it's safe and effective. For creating and evaluating new treatments, the process goes like this:
- Run an experiment. Researchers run careful experiments. A good experiment tests one thing at a time and keeps everything else the same.
- Publish research in a journal. Other researchers review the experiment design and results. If the authors ran a good experiment, the journal publishes the research.
- Run a clinical trial. Researchers start a clinical trial to find out if a treatment is safe and effective. The clinical trial has several phases, with more people enrolled in each phase.
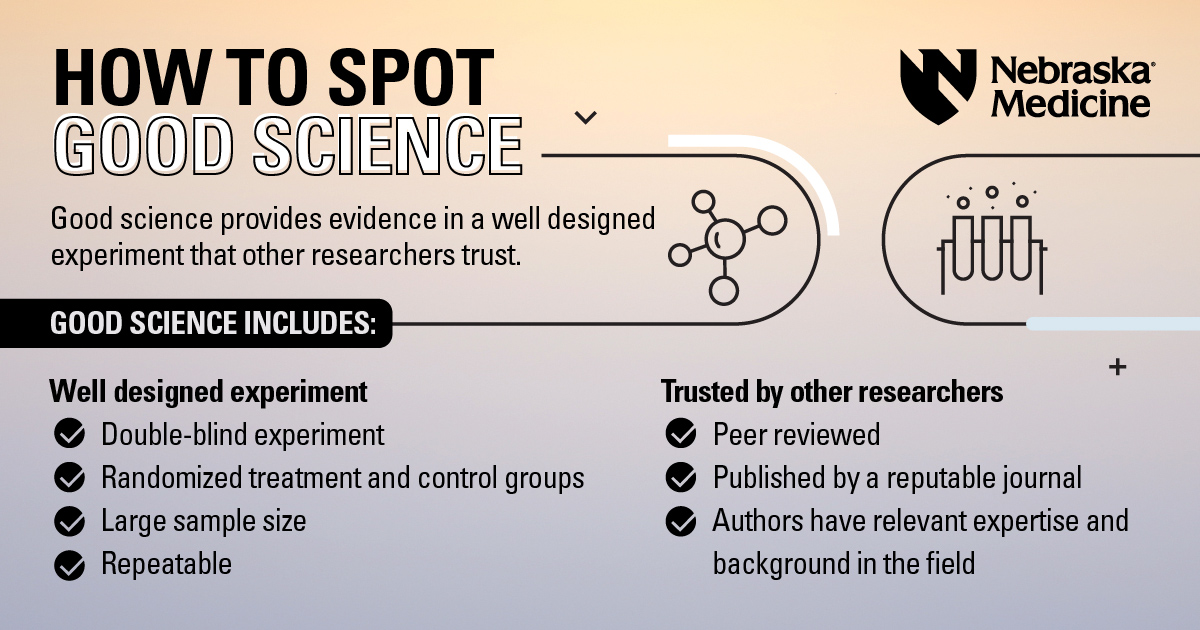
Well designed experiment
An experiment testing a potential treatment starts small – really small. First scientists look at individual cells and how they might react. Then they might study how the potential treatment performs in rats with the disease. Before a treatment gets close to a human volunteer, many well designed experiments happened first.
A gold standard experiment will be:
- Randomized: The participants are divided into at least two groups, sometimes more. One group will get a real treatment (the one that's being tested), while a control group will get a placebo* or no treatment at all
- Double-blind: Neither the researchers nor the volunteers know if they're getting a treatment or a placebo. Researchers who know a treatment is real can influence the results of the experiment, which would be bad
- Large sample size: Humans are complicated. Sex, age, diet, race, activity level, medical conditions and more all make our bodies different. A treatment that works for some people may cause severe side effects in others. The more people an experiment includes, the more likely the results will be valid
Experiments should also be repeatable. If one day you get a certain result, but the next five tries all have different results, it's probably a fluke. The same researchers should be able to repeat their results, as well as researchers from other labs.
*A placebo is a fake treatment that looks real – like a sugar pill. It has no active effect. The reason? Sometimes people feel better just from believing that they're taking a treatment. Placebos often boost recovery just through the power of our minds! With experiments involving cells and animals, no placebo is necessary.
Trusted by other researchers
If you were writing a class report as a group, and you knew someone hadn't done the reading, you wouldn't want that person to contribute and get you all an F. No, you'd want the students who had done their homework to write the report.
Similarly, only researchers who have experience in the area – who have "done the homework" – should contribute to the official learnings of the field. If it's research done on an infectious disease like COVID-19, only people with relevant expertise and training should perform the research.
Peer review is an important process for science. Peer review means other scientists who are also experts in the field review the experiment and paper. They see if the authors ran a well designed experiment. They check for errors. They make sure the authors don't overstate things.
After peer review, a journal publishes the paper. Some journals are reputable because of their strict publishing guidelines – letting only carefully performed research in. Other journals are known to let anything in, so they're less trustworthy.
The debate over hydroxychloroquine
The "white paper" that America's Frontline Doctors put out has some credibility issues.
- It was not peer reviewed
- It wasn't published in a journal of any sort – it's currently hosted on Google Drive
- The author, Simone Gold, MD, JD, is not an infectious diseases doctor. It's not known if she is currently practicing
The white paper includes references to other studies.
- Nearly all of them are not double-blind, randomized experiments, meaning bias can sway the results
- Many of them have small sample sizes of 100 or less, which makes their results difficult to apply to larger population sizes
- Some of them are not studies at all, rather news articles or recommendations that aren't credible for evaluating a scientific treatment
For these reasons, it's difficult to accept the author's advice of using hydroxychloroquine to treat COVID-19.
The two key issues with hydroxychloroquine, as with any treatment, are:
- Is it safe?
- Does it work as a treatment for COVID-19?
Safety: The FDA found serious heart-related adverse effects and even death for some COVID-19 patients who took hydroxychloroquine or chloroquine.
Effectiveness: The Solidarity Trial is an ongoing international clinical trial to find an effective COVID-19 treatment by the World Health Organization. The data found that hydroxychloroquine does not reduce mortality of hospitalized COVID-19 patients, compared with standard of care.
Because of these issues, experienced researchers are not convinced of hydroxychloroquine's usefulness in treating COVID-19. At least not yet. Clinical research studies into hydroxychloroquine are on-going and if large-scale, randomized and double-blind experiments are published that show the safety and efficacy of the drug, guidelines would change. That's the great thing about science – we learn more every day.