Were the COVID-19 vaccines rushed? Here's how the vaccines were developed so fast
In a first for vaccine development, two COVID-19 vaccines were created, evaluated and authorized for emergency use in under a year. Despite the fast timeline, these vaccines went through the appropriate clinical trials, just like other vaccines before. The CDC continues to closely monitor these vaccines for safety and efficacy.
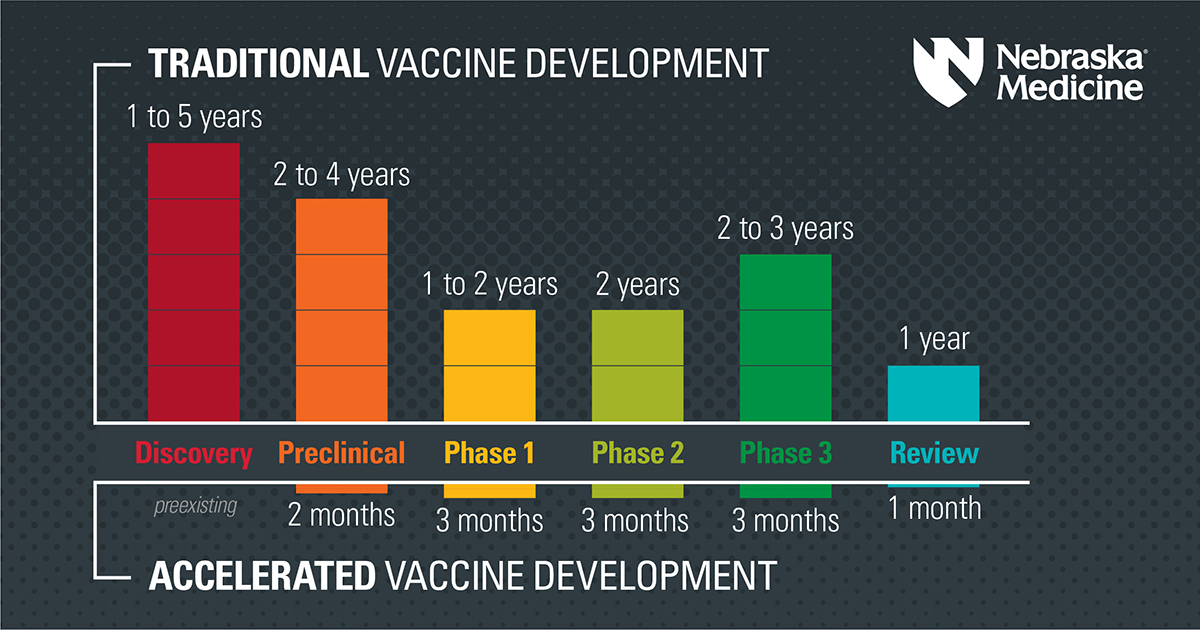
How did the vaccines get developed so quickly? The vaccine developers did not cut corners. Rather, a true global emergency paired with early application of substantial resources made it possible.
1. A smart head start
Researchers need to do a lot of academic research and pre-clinical work to make medical products such as vaccines. In this case, SARS-CoV-2 was a new virus, but it belongs to a family of viruses with similar traits. Scientists had studied other coronaviruses for 50 years. They already knew that the spike protein could be targeted by a vaccine, which gave them a goal to work toward immediately.
2. A decade of mRNA vaccine research
Researchers have been developing and researching an mRNA vaccine platform for over 10 years. After SARS-CoV-2 was sequenced, it took just a few days to make the mRNA vaccine candidates. The spike protein’s genetic code was plugged into preexisting technology with an already working process that had been evaluated for other vaccine uses, such as in the fight against dengue.
3. Participants enrolled in the trials quickly
Large scale vaccine clinical trials were organized quickly using networks established in the pursuit of an HIV vaccine. People enrolled quickly due to widespread public interest. Plus, the virus has been spreading rapidly, so regulators could see if vaccine candidates made a difference in a shorter amount of time.
4. Building facilities ahead of time
The U.S. Federal government and others pre-built manufacturing facilities for several different vaccines before knowing if they would be approved. Typically, vaccine manufacturers wait until after phase 3 has been completed, and licensure is expected to spend so much money on these types of facilities.
5. Overlapping phases
Regulators at the Food and Drug Administration and those involved in making these vaccines already had seen scientific results on the mRNA vaccine platform. So researchers could focus their questions on animal models and early human trials so that they were completed more quickly. In some instances, there was an overlap of certain study phases.
6. Expedited review
As part of its review process, the FDA re-analyzes the data that companies provide. This double-checking process is reassuring because a neutral party independently evaluates the claims made by the manufacturers. It can take six to nine months for new treatments to get through this process. The FDA compressed the review timeline to weeks with people working nights, days and weekends on parallel teams.
Risk versus reward
There are no silver bullets in medicine, and these COVID-19 vaccines are no exception.
Every medication, treatment and vaccine carries risk. Cancer patients undergo chemotherapy, which is physically and emotionally taxing because the alternative of untreated cancer can be far worse. Using antibiotics to treat a bacterial infection can cause side effects like nausea, diarrhea and cramping. However, millions of people have received the COVID-19 vaccines so far, and the sometimes unpleasant side effects appear worth the decreased risk of contracting COVID-19. Some adverse effects are more severe for people with allergies, for instance. When in doubt, people should consult with their physician. There may be additional vaccine options available over time.
The number of lives lost to COVID-19 in the United States is over 600,000. After understanding the risks and the benefits of the COVID-19 vaccine, it is ultimately your choice to decide whether you want it or not.
Regardless of your decision to get a COVID-19 vaccine, these are essential aspects of all of our work to stop the pandemic:
- Mask use
- Physical and social distancing
- Being mindful of when you or others are ill and of crowded settings
- Personal and environmental hygiene
- Participation in active screening
- Following quarantine and isolation guidelines
- Supporting others who choose to get the COVID-19 vaccines