New prostate cancer treatment provides hope
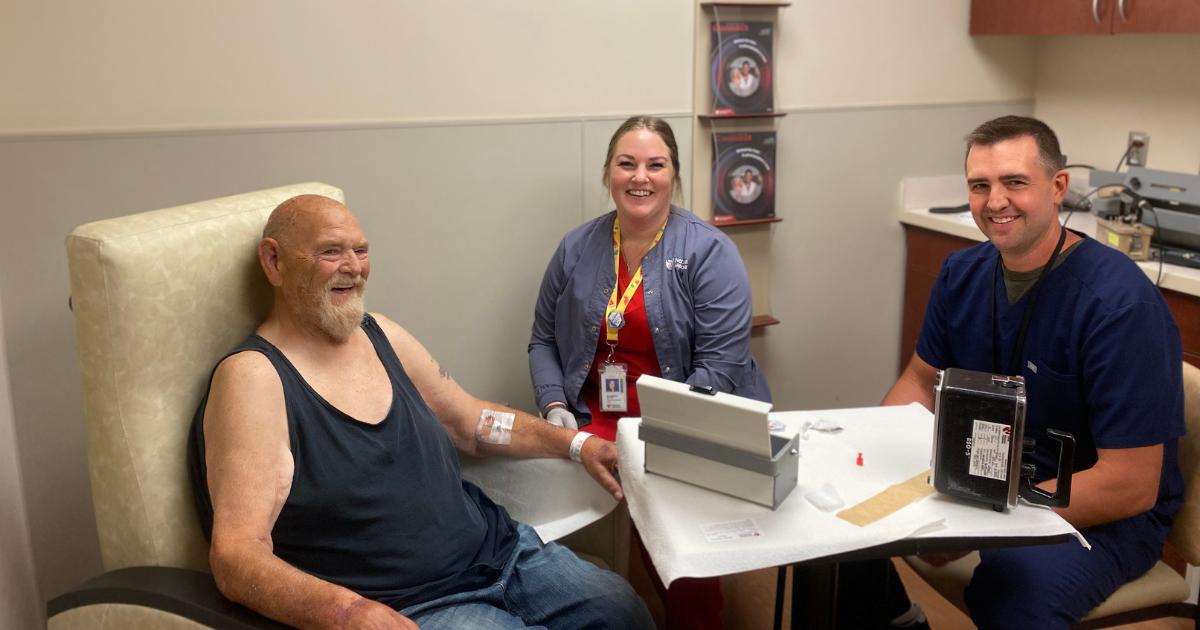
When Phil Painter learned he had stage 4 prostate cancer last year, he wasn’t sure what the future would hold.
Doctors discovered the cancer had metastasized into his bones, spine and ribs. He started chemotherapy but had a bad reaction to the treatment.
“I felt very sick after the chemo and lost my appetite,” Painter says. “Even cold water tasted bad.”
A PET/CT scan revealed he would be the perfect candidate for a new FDA-approved prostate cancer treatment called PLUVICTO (lutetium Lu 177 vipivotide tetraxetan). In August, he became the first Nebraska Medicine patient to receive this treatment.
PLUVICTO is a radiopharmaceutical designed to treat adult patients who have an advanced form of cancer called prostate-specific membrane antigen-positive metastatic castration-resistant prostate cancer, also known as PSMA+ mCRPC. The treatment is used when cancer has spread to other parts of the body and the patient has failed other traditional anticancer treatments, such as prostate removal, radiation therapy and chemotherapy.
“We will be one of a selected few sites in the states of Nebraska and Iowa that will be able to offer this treatment to patients,” says Harshraj Leuva, MBBS, Hematology/Oncology. “It is always good to have options available for patients as it allows us to offer personalized cancer care.”
Nurse Manager Erin Griesman, whose unit helps monitor patients before and after PLUVICTO injections, says this new treatment is a great resource for patients.
“This is an option that gives our patients an opportunity to have more time with their families,” Griesman says.
A study of trials from the makers of PLUVICTO found that men with PSMA+ mCRPC who received this therapy plus the best standard of care had an overall survival benefit and radiographic progression-free survival benefit compared to patients who did not get the treatment.
PLUVICTO is given to the patient as a series of 6 IV injections administered by a nuclear medicine technologist once every 6 weeks. Painter received his first dose in August and is scheduled to receive his second dose in October.
“I’m feeling good so far,” Painter says. “The nurses have all been great. I’m hoping this next treatment goes just as well as the last one.”
What makes PLUVICTO unique is that it is a form of theranostic treatment, meaning it is a precision medicine that uses radiation to target certain receptors expressed on cancer cells in the body as compared to chemotherapy, which can have effects on all cells in the body. It works by delivering radiation treatment directly to prostate-specific membrane antigen-positive (PSMA+) cells. PSMA is a biomarker that is expressed on prostate cancer cells and can be seen on a PSMA PET/CT scan. Once an injection is given, the radioactive medicine attaches to PSMA, is absorbed by the cancer cells and releases radiation that can damage and kill cells that are PSMA+.
“This is a very exciting new therapy for prostate cancer; theranostics in general are a promising future mechanism to treat different forms of cancer,” Craig Johnson, MD, Nuclear Medicine, says. “There are many current and pending theranostic clinical trials for various types of cancers in the pipeline to provide a personalized approach in cancer care.”
Nebraska Medicine is in the process of identifying other patients who may qualify for this new prostate cancer treatment and hopes to eventually provide PLUVICTO to several patients each week.